Klesh-E-Vak - Vaccine of tick-borne encephalitis, culturally purified, concentrated, inactivated, sorbed Registration number: LP 001584 Trade name: Klesh-E-Vak Group name: Vaccine for the prevention of tick-borne encephalitis Dosage form: Suspension for intramuscular administration Country of origin: FSUE "PIPVE im. . M.P. Chumakov RAMS", Russia, Moscow ATX code: J07BA01 Preliminary examination: Not required Vaccine cost: 1300 rubles (examination before vaccination is additionally paid) Applicable: For adults and children Conditions of release: only for medical and preventive and sanitary institutions .
Leave a request for vaccination. Our manager will coordinate an appointment time convenient for you.
Sign up for vaccination
Sign up by phone
+7 (495) 788-000-2
DESCRIPTION OF THE MEDICINE
The Tick-E-Vac vaccine is a purified, concentrated suspension of formalin-inactivated tick-borne encephalitis virus (TBE) strain “Sofin”, obtained by reproduction in a primary culture of chicken embryo cells sorbed on aluminum hydroxide.
Chicken embryos are obtained only from healthy birds from poultry farms that are free from infectious diseases of chickens; the quality of the supplied embryos is confirmed by veterinary certificates and certificates from the veterinary laboratory on the sanitary condition of the livestock, including microbiological and biochemical controls.
Appearance — Homogeneous suspension of white color, without foreign inclusions. Pharmacotherapeutic group - MIBP vaccine Immunological properties - The vaccine stimulates the production of cellular and humoral immunity to the tick-borne encephalitis virus. After two injections of the drug (vaccination course), virus-neutralizing antibodies are detected in at least 90 vaccinated people.
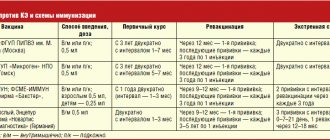
Vaccination scheme
In Russia, the two most common vaccines are “Tick-E-Vac” and “Tick-Borne Encephalitis Vaccine”. These are domestically produced drugs containing an inactivated virus. Vaccination against tick-borne encephalitis using these drugs is not capable of causing the disease. After vaccination, the human body produces specific antibodies to the injected virus, providing immunization to this type of pathogen.
There are two immunization regimens: two- and three-component. Both of them ensure the production of the required amount of antibodies to the virus, but differ in the duration of maintaining their concentration at the required level.
Two-component circuit
The first dose of the vaccine should be administered 1-3 months before the start of the high incidence season. The two-stage vaccination scheme is carried out as follows:
- 1-3 months before the season of increased risk of tick-borne encephalitis (approximately April);
- 1-7 months after the first dose.
The best effect is to plan immunization in such a way that the second injection is given 1 month before the intended trip or before the start of the season. Revaccination should be done after 1 year and then every 5 years. However, according to research, a sufficient concentration of antibodies in the body remains for 6-8 years.
The vaccine is injected intramuscularly into the upper third of the shoulder. Vaccination against tick-borne encephalitis may not be effective enough in people with congenital or acquired immunodeficiency. This group includes patients undergoing or receiving immunosuppressive drugs (chemotherapy, high doses of corticosteroids, immunosuppressive drugs), patients with HIV/AIDS (see symptoms of HIV infection, AIDS dissidents), with congenital abnormalities of the immune system.
Three-component scheme
The vaccine is administered according to a three-component scheme as follows:
- The day of administration of the first dose is designated as day “0”;
- The second dose is administered 1-3 months after the first;
- The third dose of the vaccine is administered 5-12 months after the second.
Other features of vaccination
Subsequent maintenance vaccinations in both cases are carried out every three years if the risk factors for the disease persist. If a person changes place of work or residence, there is no need for immunization.
Vaccination for children from 3 to 15 years is carried out in a reduced dosage - ½ of the adult dose. The vaccine can be administered at the same time as other vaccines. Precautions include injecting different vaccines in different places and using different syringes. Maximum immunization occurs one week after the second dose.
In this case, the doctor must determine the level of antibodies in the blood after the second dose and decide on additional maintenance vaccination. Be sure to tell your doctor if you have previously received vaccinations for Japanese encephalitis, yellow fever, or dengue fever. The presence of antibodies to the pathogens of these diseases may affect the assessment of the results of a control immunological study.
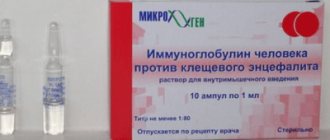
Vaccination carried out after a tick bite is ineffective. In this case, to prevent the disease, it is recommended to administer a specific immunoglobulin.
Average cost of vaccination
As for the price of vaccination, the cost of one dose of the vaccine is 400-500 rubles for Russian-made drugs and 1000-1500 for foreign ones. It is also worth considering that this is the price of only one vaccination, and 2 or 3 doses of the vaccine are required according to the scheme. As a rule, each clinic provides special offers and discounts when ordering collective immunization. Despite the difference in cost, the effectiveness of domestic and European vaccines remains approximately at the same level.
COMPOUND
The vaccine contains human albumin (10% or 20% infusion solution). The manufacturer guarantees the absence of antibodies to HIV, HIV-2, hepatitis C virus and hepatitis B virus surface antigen in the vaccine, based on the documents provided by the manufacturer of human albumin (registration certificate, analytical passport, certificate of conformity, declaration of conformity).
One vaccination dose for children from 1 year to 16 years (0.25 ml) contains:
Active components:
- Inactivated TBE virus antigen - titer not less than 1:128.
Excipients:
- Human albumin (solution for infusion 10% or 20%) - 0.125 mg;
- Sucrose -15 mg;
- Aluminum hydroxide - 0.2 mg;
- Buffer system salts: sodium chloride - 1.9 mg, trometamol-O, OZ mg.
One vaccination dose for persons 16 years of age and older (0.5 ml) contains:
Active components:
- Inactivated TBE virus antigen - titer not less than 1:128.
Excipients:
- Human albumin (solution for infusion 10% or 20%) - 0.25 mg;
- Sucrose - 30 mg;
- Aluminum hydroxide 0.4 mg;
- Buffer system salts: sodium chloride - 3.8 mg, trometamol - 0.06 mg.
Solutions for albumin infusions contain (in addition to albumin) sodium capripate and sodium chloride. The drug does not contain formaldehyde, antibiotics or preservatives.
WHO position
Immunization provides the most effective protection against tick-borne encephalitis. The vaccines used in Russia are safe and are indicated for use by people from the age of three. After their use, stable immunity develops, providing protection against most types of tick-borne encephalitis virus, characteristic of Asia and Europe.
Before mass immunization begins in each specific region, a thorough assessment of the number of cases of morbidity among the population is necessary. If the risk of morbidity is low, vaccination is considered inappropriate. It is necessary to consider vaccination if the incidence in the region is from 5 cases per 100 thousand population.
In high-risk areas, vaccination should be included in the national immunization program. In other cases, vaccination is given individually to people planning to travel to an endemic area or to rotational workers.
Author:
Nikita Aleksandrovich Korobov, anesthesiologist
INDICATIONS FOR USE
Specific prevention of tick-borne encephalitis for persons aged 16 years and older at a dose of 0.5 ml and for children from 1 year to 16 years at a dose of 0.25 ml; immunization of donors in order to obtain specific immunoglobulin.
Contingents subject to specific prevention:
- Population living in territories exotic for tick-borne encephalitis;
- Persons arriving in these territories performing the following work:
- agricultural, drainage, construction, excavation and movement of soil, procurement, fishing, geological, survey, expedition, dermatosation and pest control;
- for logging, clearing and landscaping of forests, health and recreation areas for the population.
- Persons visiting areas endemic for tick-borne encephalitis for the purpose of recreation, tourism, work in summer cottages and gardens;
- Persons working with live cultures of tick-borne encephalitis.
Who is strongly recommended to get vaccinated against tick-borne encephalitis?
It is especially recommended to get vaccinated for those people who, due to circumstances or their work, spend a lot of time in dangerous places: forests, near lakes or rivers, swamps, in the taiga. This applies to:
- field workers;
- huntsmen, foresters, beekeepers;
- amateur fishermen and/or hunters;
- summer residents;
- personnel of tourist bases in forest recreation areas and camps;
- children going to children's camps in epidemiologically dangerous areas.
The shortest period between vaccination and departure to an unsafe place is 2 weeks. But it is best to complete the vaccination procedure according to the chosen scheme a month before departure.
Galina Vladimirovna
CONTRAINDICATIONS
- Acute infectious and non-infectious diseases, chronic diseases in the acute stage - vaccinations are carried out no earlier than 1 month after recovery (remission);
- History of severe allergic reactions; bronchial asthma; autoimmune diseases;
- History of allergy to the components of the drug;
- Severe reaction (fever above 40C; swelling, hyperemia more than 8 cm in diameter at the site of vaccine administration) or complications to the previous dose of the vaccine;
- Children under 1 year.
When vaccinating donors, the contraindications listed above, as well as contraindications related to donor selection, should be taken into account.
In each case of a disease not contained in this list of contraindications, vaccination is carried out with the permission of a doctor, based on the health status of the person being vaccinated and the risk of contracting tick-borne encephalitis. In order to identify contraindications, the doctor (paramedic) conducts a survey and examination of the vaccinated person on the day of vaccination with mandatory thermometry.
Adverse reactions to vaccination against tick-borne encephalitis
There are not very many possible side effects, but a person who decides to get vaccinated should also be aware of them. This:
- allergic reaction;
- muscle pain;
- compaction at the site of vaccine administration;
- some neurological abnormalities (especially if a person has chronic neurological diseases);
- urge to vomit;
- nausea.
Some doctors believe that lactation and pregnancy are not contraindications for vaccination, others insist on the opposite. Since there is no clear answer, a mandatory consultation with a specialist is necessary.
METHOD OF APPLICATION AND DOSAGE
The drug is administered intramuscularly into the deltoid muscle of the shoulder.
PREVENTIVE VACCINATION
1.1. Routine vaccination
The primary course of vaccination consists of two intramuscular injections of 1 dose each with an interval of 1-7 months.
One vaccination dose is:
- Adults 16 years and older - 0.5 ml;
- Children from 1 to 16 years old - 0.25 ml.
Vaccinations can be carried out throughout the year, including during the epidemic season. Visiting a TBE site during the epidemic season is allowed no earlier than 2 weeks after the second vaccination.
The most optimal interval between the first and second vaccinations is 5-7 months (autumn - spring).
1.2. Emergency vaccination
For epidemic indications, emergency vaccination may be carried out. In this case, the vaccine is administered twice with an interval of 2 weeks to the following persons:
- Adults 16 years and older - 0.5 ml;
- Children from 1 to 16 years old - 0.25 ml.
Visiting the TBE outbreak during the epidemic season is allowed no earlier than 2 weeks after the second vaccination.
The first revaccination with both regimens is carried out once 1 year after completion of the primary course of vaccination with a dose of 0.5 ml for persons aged 16 years and older and a dose of 0.25 ml for children from 1 year to 16 years.
Subsequent distant revaccinations are carried out once every three years at an age-specific dosage.
GENERAL SCHEME OF VACCINATION
| Type of vaccination | First vaccination | Second vaccination | Subsequent (revaccination) |
| Routine vaccination | Selected date | 1-7 months after the first vaccination | After the second - every 3 years |
| Emergency vaccination | Selected date | 2 weeks after the first vaccination | |
| Dose for adults 16 years and older | 0.5 ml | 0.5 ml | 0.5 ml |
| Dose for children from 1 year to 16 years | 0.25 ml | 0.25 ml | 0.25 ml |
VACCINATION OF DONORS
The course of vaccination is two intramuscular injections of 0.5 ml with an interval of 5-7 months or three injections of 0.5 ml with an interval of 3-5 weeks between vaccinations. The first scheme provides the best immunization effect. Revaccination - a single dose of 0.5 ml every 6-12 months. The first blood draw from donors should be carried out 14-30 days after the vaccination course.
Features of vaccination
The localization of vaccination may differ depending on the vaccine used, although recently intramuscular injections are most often used.
As for the timing of when vaccination against tick-borne encephalitis should be done, it all depends on the specific conditions. For example, when going on a business trip to dangerous regions, it is recommended to get vaccinated. However, the risk of infection among office workers and those whose profession involves working in the field will be incomparably different. The latter are strongly recommended to undergo emergency vaccination according to indications.
No special preparations are required before vaccination. It is enough to undergo an examination by a therapist before administering the tick-borne encephalitis vaccine. If a cold is suspected, additional tests will be ordered.
SIDE EFFECTS
After administration of the vaccine, local and general reactions may develop in some cases.
For adults 16 years and older
Local reactions
- Redness, swelling, pain at the injection site;
- Development of infiltration;
- Slight enlargement of regional lymph nodes.
Local reactions may appear within 2 days after vaccination. The duration of local reactions does not exceed 3 days.
General reactions
- General malaise, headache, nausea, fever (often up to 37.5 C (weak reaction)) (occasionally - from 37.5 C to 38.5 C (average reaction)) (rarely - over 38.5 C ( strong reaction)).
General reactions can develop within 2 days after vaccination, their duration does not exceed 2 days.
For children from 1 year to 16 years old
Local reactions
- Redness, swelling, pain at the injection site;
- Development of infiltration;
- Slight enlargement of regional lymph nodes.
Local reactions may appear within 2 days after vaccination. The duration of local reactions does not exceed 3 days.
General reactions
- General malaise, headache, nausea;
- Increase in temperature (up to 37.5C (weak reaction) - often; from 37.5C to 38.5C (medium reaction) - often; above 38.5C (strong reaction) - rarely).
General reactions can develop within 3 days after vaccination, their duration does not exceed 3 days.
Local and general reactions often develop after the first vaccination. In isolated cases, vaccinations may be accompanied by the development of immediate allergic reactions, and therefore those vaccinated should be under medical supervision for 30 minutes after vaccination. Vaccination sites must be provided with anti-shock therapy.
Symptoms of tick-borne encephalitis
The first symptoms of tick-borne encephalitis develop 1-2 weeks after the bite and their symptoms resemble a cold. These include fever, headache, muscle pain, and joint aches. Symptoms persist for a week. After which most patients recover.
Approximately every third patient develops a more severe form of encephalitis, which is accompanied by:
- persistent increase in temperature to high numbers
- unbearable headaches
- pain when bending the neck
- vomiting and lethargy
If such signs occur, you should immediately consult a doctor. In severe cases, encephalitis leads to irreversible damage such as paralysis or death of the patient.
Approximately 10-12 thousand cases of tick-borne encephalitis are registered annually in the world. The highest incidence rates are observed in the Baltic countries, Slovenia and Russia. On the territory of the Russian Federation, the incidence is 2.5 per 100 thousand population, but in certain endemic areas (north, north-west) this index increases by more than 5 times.
The increasing danger of tick-borne encephalitis is explained by the emergence of cases of morbidity in areas that were not previously considered endemic (Scandinavia, Switzerland, Germany).
The virus comes from more than 100 animal species, including foxes, voles, deer, dogs, monkeys and horses. There is a possibility that the tick-borne encephalitis virus can penetrate the milk from the body of infected cows. When consuming unpasteurized milk, a person becomes infected through the nutritional route.
The disease is characterized by two periods of increased incidence: May-June and August-September. Immunization of the population is planned based on this time frame.
Endemic regions for the incidence of tick-borne encephalitis are rural areas of Russia, Central and Eastern Europe and Japan. It is recommended that residents of these regions, as well as tourists and workers traveling to these areas, be vaccinated against tick-borne encephalitis. The risk of contracting tick-borne encephalitis increases during periods of high tick activity (from April to October).
In these areas, tick-borne encephalitis represents a serious economic problem due to the need for long-term treatment of neurological complications. The use of vaccination in European countries over the course of ten years has reduced economic losses by $80 million.
SPECIAL INSTRUCTIONS
The drug cannot be administered intravenously!
Vaccinations are carried out in strict compliance with the rules of asepsis and antiseptics. The room must be equipped with anti-shock and anti-allergic therapy. Before opening the ampoule, it is necessary to carry out a visual inspection. The drug is not suitable in ampoules with damaged integrity, labeling, if foreign inclusions are detected, if there are large unbreakable conglomerates, if the expiration date has expired, if the temperature conditions of storage or transportation are violated. Immediately before injection, the vaccine in the ampoule is shaken until a homogeneous suspension is obtained. The drug is administered immediately after opening the ampoule intramuscularly into the deltoid muscle of the shoulder. Vaccinations performed are recorded in established registration forms indicating the name of the drug, date of vaccination, dose, batch number, manufacturer, reaction to vaccination.
Vaccination of children and adults with chronic diseases in the acute stage is carried out no earlier than 1 month after recovery (remission) . The vaccine is not used for children under 1 year of age.