General information about the immunochip for borreliosis
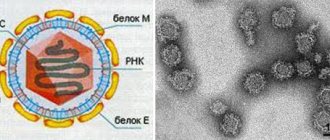
An immunochip is understood as a diagnostic test system that detects in blood serum a spectrum of antibodies to significant immune proteins of Borrelia classes M (IgM), G (IgG). This is one of the methods for diagnosing borreliosis that belongs to the category of serological differential studies. The immunochip consists of the following elements:
- Immunosorbent in the form of a glass or plastic slide coated with nitrocellulose. Antigens or synthetic peptides are separately immobilized on it. These are auxiliary controls, which are antibodies to immunoglobulin IgM or IgG.
- Conjugate, reagents. When performing an immunochip, they help identify the antigen-antibody complex.
The immunochip for borreliosis detects antibodies to the Borrelia gharinium and Afzelia antigens. They are determined by the degree of intensity of fluorescence signals (glow) at the sites of localization of a certain antigen on the immunosorbent. Today, the immunochip is considered one of the most accurate studies confirming Lyme disease. The estimated price of the analysis is about 700 rubles.
Etiology (causes) of Lyme disease
The causative agent is a gram-negative spirochete of the Borrelia burgdorferi sensu lato complex of the Spirochaetaceae family of the genus Borreliae. B. burgdorferi is the largest of the Borrelia: its length is 10–30 µm, diameter is about 0.2–0.25 µm.
She is able to actively move with the help of flagella. The microbial cell consists of a protoplasmic cylinder, which is surrounded by a three-layer cell membrane containing thermostable LPS with endotoxin properties. There are three groups of Borrelia antigens: surface (OspA, OspB, OspD, OspE and OspF), flagellar and cytoplasmic.
Borrelia are grown on a specially created liquid nutrient medium enriched with amino acids, vitamins, bovine and rabbit serum albumin and other substances (BSK medium).
Based on molecular genetics methods, more than ten genomic groups of Borrelia belonging to the Borrelia burgdorferi sensu lato complex have been identified. B. burgdorferi sensu stricto, B. garinii and B. afzelii are pathogenic for humans. The division of pathogens into genomic groups is of clinical importance. Thus, B. burgdorferi sensu stricto is associated with predominant damage to the joints, B. garinii - with the development of meningoradiculitis, B. afzelii - with skin lesions.
Borrelia are not stable in the environment: they die when they dry out; preserves well at low temperatures; at a temperature of 50 °C they die within 10 minutes; die under the influence of ultraviolet irradiation.
Basic methods of laboratory diagnostics
The causative agents of the disease are Borrelia - pathogenic organisms from the genus of spirochetes. The incubation period lasts from 2 days to a month (on average 3-11 days), after which patients experience symptoms reminiscent of influenza or ARVI, in combination with skin manifestations - annular erythema.
Lyme disease can be detected immediately after pathogens enter the body if an intact tick can be removed from the victim’s body and the insect can be delivered to the laboratory for examination using the PCR (polymerase chain reaction) method. If borrelia are detected in the DNA of the parasite, the patient is given preventive treatment with antibacterial drugs.
When symptoms of Lyme disease appear at any stage, a number of studies are prescribed to clarify the diagnosis:
- ELISA (enzyme-linked immunosorbent assay);
- chemiluminescent immunoassay;
- immunochip;
- Western blot (immunoblot);
- PCR of cerebrospinal or joint fluid.
If the disease has progressed to the second or third stage, patients are additionally prescribed a general blood test to identify inflammatory processes in the body and instrumental research methods (ultrasound, X-ray, CT and MRI), which allow assessing the condition of the internal organs and identifying possible lesions.
Materials and methods
Patient groups studied.
The study was conducted on the basis of LLC MO "New Hospital" of Yekaterinburg during the epidemic season (June-July) 2010, 2011, 2015 and 2016.
The criterion for inclusion in the study was suspicion of a tick-borne infection. We used both standard diagnostic methods (determination of antibodies - AT - IgM and IgG to Borrelia and tick-borne encephalitis virus - TBE), and original methods of specific polymerase chain reactions (PCR) detecting B
.
miyamotoi
or
B.
_
burgdorferi s
.
l .,
described in detail earlier [1, 2].
ICD-BM was diagnosed based on detection of B
.
miyamotoi
in the patient's blood in the absence of laboratory signs of other infections.
A total of 117 cases of ICD-BM were identified; 10 cases of suspected mixed B
.
miyamotoi
and TBE virus are not considered in this work.
At the same time, 53 cases of ICD-BM in 2015-2016. additionally confirmed by identifying specific antibodies (see below). Diagnosis L.B. were established on the basis of typical erythema migrans (EM) measuring more than 5 cm, which is allowed by domestic and international diagnostic standards [16, 17].
71 patients were randomly selected
the
comparison group (patients with LB, monoinfection with B. burgdorferi s
.
l Moreover, in 15 of them, the diagnosis of LB was confirmed by PCR, and in 52, antibodies to Borrelia were detected using a registered commercial test system (Euroimmun, Germany).
The compared groups are comparable by gender and age. The proportion of men among patients with ICD-BM (59%) is statistically insignificantly higher than among patients with LB (45%; p
=0.072;
Fisher's criterion). The mean age (median) in patients with LB (60 years) is higher than in patients with ICD-BM (57 years; p
= 0.051; Mann-Whitney test). In general, the age of the patients ranged from 15 to 88 years.
Methods for detecting specific antibodies to
B. miyamotoi.
In species of the group
B. burgdorferi sl.
and
B. miyamotoi
have several common membrane protein antigens, as a result of which registered test systems are not able to differentiate the immune response to these infections.
However, the GlpQ enzyme is not synthesized by B. burgdorferi sl,
but is found in
B. miyamotoi
.
It was recently shown that B. miyamotoi
is also capable of expressing a number of highly immunogenic variable surface proteins (variable major proteins—VMPs), in particular the Vlp15/16, Vlp18, Vsp1, and Vlp5 proteins [18].
The planar protein biochip developed at the Central Research Institute of Epidemiology includes antigen proteins such as B. burgdorferi sl
(
B. afzelii
- p100, VlsE, p39, p41, p58, BBK32, OspC, p17;
B. garinii
- p100, VlsE, p41, BBK32, OspC , p17) and
B. miyamotoi
(GlpQ, Vlp15/16, Vlp18, Vsp1, Vlp5), for which genetically engineered constructs encoding an antigenic region, a protein fragment, or the complete protein sequence were obtained.
Antigens were expressed in Escherichia coli
and purified by affinity and ion exchange chromatography. Immunochips were produced on slides with aldehyde coating 3D-Aldehyde Glass Slides (PolyAn, Germany) using a plotter for non-contact piezo printing S3 (Scienion AG, Germany). Accounting of the analysis results after applying the patient's blood serum and secondary antibodies to human immunoglobulins to the immunochip was carried out using a MarS multichannel fluorescent scanner (Ditabis, Germany), and the calculation, standardization and interpretation of the results were carried out using specially developed StarSky software. The level of specific IgM and IgG antibodies was characterized in a semi-quantitative manner by the value of the standardized optical signal (SOS). Based on the study of sera from healthy donors and patients with other diseases, SOS of more than 5 conventional units (arb. units) was considered to exceed the threshold level, which indicates the presence of the studied antibodies in the sample [19].
Quantitative PCR.
The bacterial load in the blood of patients with ICD-BM was measured in copies per 1 ml using quantitative real-time PCR amplifying 16S RNA or DNA of the
16 S
RNA gene, using calibration curves with titration of the corresponding targets from 107 to 103 copies per 1 ml [1 , 2].
Statistical methods.
Epidemiological and clinical laboratory data were stored in a database with more than 100 fields per record (patient). All statistical calculations and evaluations were carried out using the IBM SPSS Statistics 19 licensed program [20]. To assess the significance of differences in the distributions of quantitative and qualitative variables in groups, standard nonparametric methods were used [21]. Results are presented as medians and interquartile ranges (in parentheses).
Rules for preparing for research
IgM antibodies to borreliosis appear first, but at about 2-3 weeks from the onset of the disease. IgG immunoglobulins in the blood will be observed a little later. It can appear either after a few weeks or after a few months. For this reason, if the immunochip shows a negative result, a recheck is required after 2-4 weeks. Preparation for analysis for borreliosis is as follows:
- Avoiding physical and stressful activities.
- Avoid fatty foods and alcohol 72 hours before the test.
- You should not smoke before the test.
- Take your last meal 12 hours before the test.
- A few days before the test, in consultation with your doctor, you must stop taking medications.
When to get tested for borreliosis
Borreliosis (or Lyme disease - it was named after the place where it was first diagnosed in 1975) is a systemic pathology caused by the bacterium Borrelia Burgdorferi from the family of spirochetes. It is carried by many animals: dogs, cows, horses, sheep, birds. But it is usually transmitted to humans by the bite of an ixodid tick (it is also a carrier of encephalitis). Borreliosis has three stages of development:
- Fever, severe redness of the skin due to dilation of the capillaries (erythema annulare) - begins about a week after infection.
- Cardiac dysfunction, neuropathy, facial muscle paralysis – a month after infection.
- Joint damage - this stage can occur either several months or several years after infection.
Important. In the early stages, borreliosis can be successfully treated with antibiotics. If the disease is neglected, it can become chronic.
Therefore, it is necessary to diagnose the pathology as early as possible
If the disease is neglected, it can become chronic. Therefore, it is necessary to diagnose the pathology as early as possible.
You should get tested for borreliosis after a tick bite. Not all ticks are “infectious” (only ixodid ticks), but it is impossible to determine visually whether an insect is a carrier of the bacterium. Therefore, a “bitten” patient should consult a doctor as soon as possible. If you remove a tick from your body yourself, you should place it in a jar with a lid and take it with you to the doctor for analysis. Or entrust the procedure of getting rid of the parasite directly to a doctor.
When delivering the parasite to a medical facility, the following rules should be followed:
- Both live and dead ticks are suitable for analysis;
- If it is not possible to immediately deliver a live tick to the clinic, it is stored in a closed jar in the refrigerator;
- Place a wet cotton swab in a container with a dead tick - this will preserve the virus as much as possible;
- It is necessary to deliver the pest for analysis no later than 2 days - then the result of the study may turn out to be unreliable.
The patient should also be tested for borreliosis.
It is important to consider that the incubation period of the disease is about 14 days. Therefore, the results of studies conducted earlier than this period will be unreliable. Important
Venous blood is usually required for the initial diagnosis of borreliosis.
Important. For the initial diagnosis of borreliosis, venous blood is usually required. Preparation for the study is simple
Blood is donated on an empty stomach. You can smoke no later than 30-60 minutes before the procedure. On the eve of the study, alcohol is excluded
Preparing for the study is simple. Blood is donated on an empty stomach. You can smoke no later than 30-60 minutes before the procedure. On the eve of the study, alcohol is excluded.
It is imperative to get tested for tick-borne borreliosis when the following symptoms are observed after a tick bite:
- ring-shaped redness in the area of the bite with clear edges, which expands concentrically every day;
- febrile state, increased temperature (as at the onset of the flu);
- nausea;
- weakness;
- a sore throat;
- joint and muscle pain;
- peeling and pigmentation of the skin;
- enlarged lymph nodes;
- headache;
- photophobia;
- irritability;
- insomnia;
- disorders of concentration and memory;
- coordination problems;
- conjunctivitis;
- hives.
Analysis for borreliosis can be:
- straight;
- indirect.
Direct analysis involves identifying the Borrelia themselves. Since they live only in some tissues of the body, collecting material is fraught with some difficulties. Therefore, for primary diagnosis, indirect methods (serological) are more often used to detect antibodies to borreliosis in the patient’s blood. The most common tests are for IgG and IgG antibodies.
Antibodies of the IgM class are detected in the patient’s blood 2-4 weeks after the suspected infection and are present in it for several months. Therefore, this test is suitable for detecting recently developed borreliosis.
IgG class antibodies appear in the blood after 4-6 months and can remain in it for several years. Analysis for these antibodies allows you to identify a long-developed or already suffered disease.
A direct test (usually a PCR - polymerase chain reaction test) is carried out if the serological procedure gives a negative result, but the patient has symptoms of the disease. The patient's cerebrospinal fluid is the material being studied. A tick delivered by a patient to a medical facility is also subjected to PCR analysis.
How to identify Lyme disease in humans
Laboratory tests are the most informative way to identify the causative agents of Lyme disease in the body before the development of severe symptoms and complications.
Most often, so-called serological diagnostic methods are used for diagnosis, which make it possible to determine antibodies (immunoglobulin) in the body that are produced by the immune system after the pathogen enters the bloodstream.
We suggest you read: Borreliosis: treatment of different forms, where it is treated, types of prevention
There are several types of antibodies to borreliosis, each of which is produced at a certain stage of the disease:
- an increased concentration (titer) of IgM immunoglobulins indicates an acute course of the infectious process, since they are produced 2-4 weeks after infection;
- IgG immunoglobulins are observed at the second or third stages of the disease, and the long-term presence of antibodies to Borrelia of this class in the blood indicates the transition of the pathological process to a chronic course.
The test is taken 2 weeks after contact with the parasite, and then repeated 20-30 days later, since not all patients develop antibodies to Borrelia in the early stages.
If it is impossible to conduct a serological test for any reason, microbiological diagnostic methods, most often PCR, are used for diagnosis. They give a less accurate picture, since it is more difficult to isolate the pathogen from blood and other samples using such a study.
If venous blood is used for the study, it is taken on an outpatient basis, after which the patient can go about his business. Collecting cerebrospinal fluid and joint fluid is a complex and sometimes painful procedure, so hospitalization is required for the study.
In order for the analysis result to be as accurate as possible, before the procedure you need to prepare to adhere to the following rules:
- avoid stressful situations and excessive physical activity;
- do not consume fatty and spicy foods, as well as alcoholic beverages 72 hours before blood sampling;
- do not smoke several hours before the test;
- The last meal should be no earlier than 8-10 hours before the delivery of the biomaterial.
In addition, the doctor must be informed about all medications that the patient is taking, as well as about existing diseases.
Tests to detect Lyme disease can be taken at any private clinic; the approximate cost is 600-1000 rubles, depending on the diagnostic method. In public hospitals, the study is carried out free of charge, but not all medical institutions have the necessary equipment and chemicals for this.
For serological diagnosis by ELISA, venous blood is used, which is taken 2-4 weeks after the bite, and then repeated 20-30 days later. The study provides a fairly informative picture of the disease, but sometimes requires additional tests to clarify the diagnosis.
Interpretation of a blood test using ELISA for borreliosis
| Antibodies to Borrelia | Negative result, OU/ml | Doubtful result, OU/ml | Positive result, OU/ml |
| Anti-Borrelia IgM | less than 0.8 | 0,8-1,1 | more than 1.1 |
| Anti-Borrelia IgG | less than 16 | 16-22 | more than 22 |
Indicators, or reference values, in deciphering the results of a blood test for Lyme disease may differ depending on the laboratory, so you should consult a doctor to make an accurate diagnosis.
Immunochip
The immunochip technology is similar to the ELISA method, and is based on identifying antibodies in the patient’s blood that are produced when Borrelia enters the body. The advantages of the technique are that it allows you to get results much faster, and also has not two, but many more markers, therefore it determines the presence or absence of the disease with greater accuracy.
We suggest you read: Tick-borne borreliosis or Lyme disease
In addition to serological studies, if borreliosis is suspected, bacteriological (cultural) methods are used, which involve isolating the pathogen on nutrient media.
Their value in making a diagnosis is quite low, since Borrelia are very demanding on the conditions of the analysis, and the likelihood of obtaining a false result increases.
Decoding the results of such studies is simple - depending on the presence or absence of pathogens in the biomaterial, the analysis can be positive or negative.
At the second and third stages of the disease, patients are prescribed a general blood test, which allows identifying inflammatory and infectious processes in the body - characteristic markers for borreliosis are an increase in ESR and an increase in the concentration of leukocytes.
Blood tests are especially important in diagnosing Miyamoto's borreliosis, which occurs without annular erythrema, a specific symptom of Lyme disease, which makes it much more difficult to identify. In this case, patients require a comprehensive diagnosis, which includes various research methods and careful monitoring of their health status.
The PCR technique is based on the detection of Borrelia DNA fragments in the patient’s biological fluids (blood, cerebrospinal fluid) and tissues from the affected area. The analysis has a number of advantages, including accessibility, high accuracy of results and speed of obtaining them - the maximum period is 72 hours.
In addition, using PCR analysis, it is possible to identify not only Borrelia, but also other pathogenic microorganisms dangerous to health. The transcript of the study after a tick bite is as follows: a positive or negative result, depending on the presence of Borrelia DNA in the samples.
Immunoblot and Western blot are among the most informative and modern research methods, which involve the identification of specific protein compounds in the patient’s biomaterial.
They make it possible to identify antibodies to 10 antigens of pathogenic microorganisms during diagnosis, determine its stage and chronic course. The analysis is done 2-4 weeks after the bite, after which it is repeated a month later to clarify the diagnosis and assess the dynamics of indicators.
Most often, Western blot and immunoblot are used for the diagnosis of tick-borne borreliosis in combination with serological research methods.
The disadvantage of these methods is that they often give a false negative result, that is, they show the absence of the disease in infected people, which can lead to incorrect diagnosis and lack of adequate treatment. Most often, this phenomenon is observed in people with immunodeficiency states - their body is unable to give an adequate response to the presence of the pathogen, which is why specific immune complexes are absent in the blood.
If the entire tick was removed from the body of the injured person, it is taken to the laboratory for examination for the presence of borreliosis pathogens. For this, the PCR method is used, which makes it possible to identify the genetic material of Borrelia in the DNA of the parasite. Infection of a tick does not 100% indicate that the bitten person was infected with the disease, but to prevent unpleasant consequences, he is placed under strict medical control and preventively treated with antibacterial drugs.
We invite you to familiarize yourself with: Tick-borne borreliosis code ICD
results
From the anamnesis of 116 out of 117 patients with ICD-BM and 68 out of 71 patients with LB, it is reliably known that the disease arose after sucking on an ixodid tick. The tick was removed on the day of suction (65%) in 75 patients with ICD-BM and 36 (52%) patients with LB ( p
=0.007).
The incubation period was slightly longer for IKB-BM than for LB: 14 (11–17) days for IKB-BM and 12 (8–19 days) for LB ( p
= 0.02).
The onset of ICD-BM was acute and patients were hospitalized on average the next day (from 1 to 2 days) after the onset of clinical symptoms, patients with LB on the 4th day (from 2 to 13 days; p
= 10–14).
Inpatient treatment with ICB-BM is longer than treatment with LB: 13 (11–15) and 10 (10–11) days, respectively ( p
= 10–14). Clinical manifestations of febrile syndrome are typical for ICD-BM, which are rarely found in patients with LB (Table 1);
Table 1. Frequency of detection of clinical symptoms in the examined patients with ICD Note. Data are presented as median (interquartile range), * - per patient, out of 22 registered symptoms, the presence of ME is not taken into account. 37% of patients with ICD-BM have more than 4 corresponding symptoms of the disease. ME in ICD-BM is practically absent, therefore, previously this disease was clinically classified as ICD in an erythematous form. The presence of ME in 5% of cases of ICD-BM probably indicates a mixed infection of B. miyamotoi
and
B. burgdorferi sl
. On the contrary, only one patient with ICD in the non-erythema form had
B. burgdorferi sl DNA found in the blood,
which became the basis make a diagnosis of LB in a generalized form.
The main quantitative laboratory parameters that distinguish IKB-BM from LB are given in Table. 2.
Table 2. Laboratory examination data of patients in the midst of ICD Note.
* — the proportion of patients whose indicator value was above (or below) the given limit is indicated; ** - definition and rules for calculating CPT are given in the text. Other general clinical and biochemical parameters of blood and urine did not go beyond the physiological norm or did not differ statistically significantly in the study group and the comparison group, and are not shown in the table. GFR—glomerular filtration rate; ALT—alanine aminotransferase; CRP - C-reactive protein; CBT is a comprehensive measure of severity. In a comparative aspect, several types of symptoms can be distinguished. Firstly, the immediate manifestations of fever and a generalized inflammatory reaction are high body temperature, increased heart rate, high levels of CRP, the appearance of band neutrophils in the peripheral blood, and partly proteinuria. Secondly, leukopenia (due to lymphopenia in the absence of neutrophilic pleocytosis) and lymphopenia itself, granulocytopenia and thrombocytopenia, which probably indicates the activation and “consumption” of these blood cells during B. miyamotoi
.
Thirdly, the initial signs of renal dysfunction are an increase in creatinine concentration and, as a more accurate indicator, a decrease in GFR (CKD-EPI) based on creatinine, calculated with adjustment for the sex and age of patients. Fourthly, in almost 50% of patients with ICD-BM, the concentration of liver enzymes in the blood, primarily ALT, increases. It should be noted that disorders in these groups of indicators are apparently independent and do not correlate with each other or with the severity of clinical manifestations of the disease. With the exception of obvious connections (proportion and number of cells, etc.), statistically significant, although weak, correlations were noted only for body temperature and the level of CRP (Spearman correlation coefficient - CRR = 0.32), CRP and the level of protein in the urine (CRR =0.47), number of platelets and lymphocytes (CCR=0.24; p
<0.05).
Since the pathological manifestations do not duplicate, but complement each other, their combined effect can be reflected on a certain scale of the complex severity index (CSI) of borreliosis. To construct the scale, the following 13 indicators were used: body temperature, heart rate, leukocyte count, neutrophil count, proportion of band neutrophils, lymphocyte count, platelet count, blood creatinine level, GFR, ALT level, CRP concentration, proteinuria (see Table 2) and “total number of disease symptoms” (see Table 1). If the values of the indicator in a particular patient went beyond the reference values indicated in the table. 1, 2, into the pathological area, then its contribution to CPT was assessed as 1. (the exception was body temperature, which was considered high, contribution - 1 for values above 39.2 ° C).
The average CPT for ICD-BM (5.7±1.9) was 5 times higher than the CPT for LB (1.1±1.1). Borreliosis with an increased severity of the course will further be considered a disease with SPT 5 or higher: 71% of cases of ICD-BM and 1 of 71 cases of LB are classified as this (see Table 2).
Concentration of DNA and RNA of B. miyamotoi
measured by the
16 S
RNA gene in the blood of 19 patients with ICD-BM upon admission to the hospital and over a period of 5 days.
As an indicator of the level of bacteremia, only the DNA/RNA concentration upon admission will be used further, since during treatment it drops sharply and on the 3-4th day in the hospital it is determined only in 2 patients, and on the 5th day it is not detected. It is more correct to analyze the average value and range of concentrations and present them in logarithmic units, since the distribution of concentrations is highly skewed (not Gaussian), and the distribution is Log10[kon, bell-shaped. The median Log10[DNA] at admission is 4.0 (3.4—4.3), i.e., the DNA concentration is 10,000 copies/ml; median Log10[RNA] at admission was 4.3 (3.3–4.8). B. miyamotoi
RNA levels in the blood ranges from 0 to 420,000 copies/ml. The amount of RNA correlates with the amount of DNA (KCR = 0.83), but on average is 2.5 times higher than the amount of DNA. This indicates that before taking the sample, at the time the patient was admitted, Borrelia multiplied, although not too actively.
DNA (and RNA) concentration of B. miyamotoi
in the blood inversely correlates with the incubation period (CCR = –0.55) and the length of the time interval between the onset of the disease and hospitalization (CCR = –0.40), i.e. spirochetemia is maximum when the disease develops relatively quickly (Fig. 1, A).
Rice. 1. Relationship of bacterial load in the blood with epidemiological and laboratory parameters in ICD-BM. The concentration of B. miyamotoi DNA in the blood at the height of ICD-BM is expressed in genome copies per 1 ml and is plotted on a logarithmic scale as Log10[DNA concentration]. a - two graphs are combined - the dependence of Log10[DNA concentration] on the incubation period of the disease (circles) and the interval between the onset of symptoms before hospitalization (squares); individual points correspond to the values recorded for each of the 19 patients studied; solid lines represent an approximation of the relationship between variables along the X and Y axes, performed by the least quadrant method with local weighting by the Epanechnikov kernel function. Horizontal dashed lines (b-h) correspond to the boundary between “normal” (reference) and “pathological” values of indicators plotted on the Y axis. The concentration of B. miyamotoi
correlates with body temperature in the hospital (KCR = 0.34), heart rate at admission (CCR = 0.60) and the total number of clinical symptoms of the disease (CCR = 0.48), i.e. with immediate manifestations of febrile syndrome (see Fig. 1, b-d).
It was unexpected that at a high concentration of B. miyamotoi
, thrombocytopenia (CMR = 0.51) and neutropenia (CMR = 0.51) are less pronounced, the level of creatinine in the blood (CMR = –0.38) and protein in the urine (UCR) are lower =–0.43) (see Fig. 1, e-h).
Probably, these observations are explained by the fact that at the peak of spirochetemia these disorders do not yet have time to manifest themselves, and in the process of interaction of the immune system with the pathogen, not only the concentration of borrelia in the blood decreases, but also the number of effector blood cells decreases, and organ pathology develops. Due to the multidirectional relationships between the level of spirochetemia and the pathological manifestations of ICD-BM, no correlations between the concentration of DNA or RNA of B. miyamotoi
and SPT were found.
The concentration of specific IgM and IgG was measured twice: upon admission of a patient with ICD-BM (on average on the 2nd day of the disease) and upon discharge (on average on the 14th day of the disease). Although the immunochip has been used to evaluate the immune response to a wide range of antigens (see section “Materials and Methods”), of which the greatest interest is caused by antibodies to the main variable surface proteins (VMPs) of various families (Vlp15/16, Vlp18, Vsp1 and Vlp5), in this publication, for simplicity, the response to VMPs will be characterized by the largest response to 1 of the 4 VMPs. In addition, the response to GlpQ, a specific enzyme of Borrelia, the causative agent of CVL, will be considered.
Upon admission, only 4 out of 32 examined patients with ICD-BM had IgM antibodies to GlpQ, and only 1 patient had IgG (and IgM) antibodies (Fig. 2).
Rice.
2. Concentration of specific IgM and IgG antibodies against GlpQ and VMPs in the blood of patients with ICD-BM during hospitalization (a, b) and discharge from hospital (c, d). The concentration of IgM and IgG antibodies against GlpQ and VMPs was measured using an immunochip and expressed in arbitrary units based on the SEM value. Horizontal and vertical dashed lines correspond to the thresholds between SES values indicating the reliable presence of AT, and SES values in the absence or low level of AT. Individual points correspond to the values recorded for each of the 32 patients studied: 21 patients with a more severe course of ICD-BM (white circles) and 11 patients with a less severe course (black circles) In Fig. a, d and especially b, many points lie in the area corresponding to the absence of AT (lower left quadrant) and partly overlap each other. The arrows indicate the data of patients admitted on the 29th and 44th days after tick ingestion, probably during a relapse of ITB-BM. It is noteworthy that this patient was hospitalized 29 days after tick ingestion, and 18 days earlier he had a history of serious illness, probably provoked by the first episode of ICD-BM, but diagnosed as a somatic illness. The immune response to surface antigens of VMPs develops earlier; upon admission, the level of IgM and IgG antibodies to VMP exceeded the threshold in 10 and 4 patients, respectively. At discharge, 26 and 28 patients had IgM antibodies to GlpQ and VMPs, respectively. Only 1 patient out of 32 lacked IgM antibodies to both GlpQ and VMPs. IgG antibodies to VMPs and GlpQ were produced in 19 and 14 patients, respectively (see Fig. 2); in 11 (34%) patients specific antibodies were not yet detected. (According to our data, the maximum level of IgG antibodies to B. miyamotoi
is achieved 30-90 days after ITB-BM disease.)
At discharge, the level of specific antibodies varied widely: from 5 conventional. units (threshold value) up to 50 conventional units. by SOS value. Statistically significant correlations of AT levels with individual characteristics of the acute phase of the disease, including the indicators listed in Table. 1, 2, and the concentration of DNA and RNA of B. miyamotoi
upon admission, not detected.
Moreover, 21 (66%) of 32 patients had increased severity of ICD-BM. In this subgroup of patients (see Fig. 2, c, d, white circles), the level of IgM to VMPs and GlpQ is lower than in the subgroup of 11 patients with a less severe course ( p
= 0.005 and
p
= 0.05 according to the Mann-Whitney test respectively). Levels of specific IgG at discharge did not differ between these subgroups.
False positive result
| Antibody type | Type of immunochip result | Interpretation criterion |
| G (IgG) | Negative | The positivity rates for Borrelia afzelia and garinia are less than 1.1. |
| Positivity rates for Borrelia afzelii and Borrelia garinii p17 antigens are greater than or equal to 1.1. | ||
| Positive | Positivity rates for Borrelia afzelii and Borrelia garinii antigens are equal to or greater than 1.1. | |
| The positivity rate for Borrelia afzelii and/or Borrelia garinii VlsE antigens is greater than 1.1 (in combination with or without antibodies to other borreliosis antigens with a positivity rate greater than or equal to 1.1). | ||
| The positivity rate is greater than or equal to 1.1 for Borrelia afzelii antigen and/or for Borrelia garinii p100, p39, p41, Bbk32, p58, OspC. | ||
| M (IgM) | Negative | Positivity rates for Borrelia afzelii and Borrelia garinii are less than 1.1. |
| Positive | Positivity rates for Borrelia afzelii and Borrelia garinii antigens are equal to or greater than 1.1. | |
| The positivity rate is greater than or equal to 1.1 for the OspC antigen of Borrelia afzelii and/or for Borrelia garinii p41, p17, VlsE. |
The probability of obtaining a false positive result is 5-10% even when using modern diagnostic techniques. In such cases, tests show the presence of pathogenic microorganisms in people who have not been infected with Borrelia.
Typically, false-positive results are observed when the patient has other infections caused by the activity of spirochetes (for example, syphilis), but sometimes their cause remains unclear.
Advantages of the method
An immunochip for determining borreliosis has several advantages over others, including serological tests for this disease. The main advantages include:
- the ability to determine a wide range of antibodies of classes G (IgG), M (IgM) to various types of bacteria that cause borreliosis;
- accuracy of determining the amount of antibodies;
- the ability to evaluate not only the qualitative composition of antibodies, but also their quantity based on the positivity rate;
- the ability to identify the duration of infection with borreliosis;
- high sensitivity;
- ease of execution;
- automated interpretation of results, which eliminates the subjectivity of the assessment;
- A small amount of the test material is taken for analysis.
Treatment
The basis of treatment for tick-borne borreliosis is antibiotics - drugs of a number of penicillins, cephalosporins, macrolides, as well as broad-spectrum agents.
In combination with antibacterial therapy, symptomatic therapy is used, which can reduce the manifestations of the disease and alleviate the patient’s condition - non-steroidal anti-inflammatory drugs, antihistamines and antipyretic drugs, immunomodulators, vitamin complexes. The treatment regimen and features are determined by the doctor depending on the clinical course of the disease and other factors.
Lyme disease treatment
Mode. Diet
The patient’s activity mode is determined by the severity of the disease:
ward mode - for mild, moderate course of the disease; bed rest - for severe cases, myocarditis, cardiac arrhythmias, meningoencephalitis, polyarthritis.
No special diet is required for patients (table No. 15).
Drug treatment for Lyme disease
The basis of treatment is antibacterial drugs, the dose and duration of administration of which are determined by the stage and form of the disease (Table 17-44).
Timely initiation of treatment promotes rapid recovery and prevents the process from becoming chronic.
Table 17-44. Antibiotic treatment regimens for Lyme disease
| Character of the current | Form | A drug | Single dose | Method of administration | Reception frequency | Duration, days |
| Acute | Stage of early localized infection | The main drug is doxycycline | 0.1 g | Inside | 2 | 10 |
| Drugs of choice | ||||||
| Amoxicillin | 0.5 g | Inside | 3 | 10 | ||
| Cefixime | 0.4 g | Inside | 1 | 10 | ||
| Azithromycin | 0.5 g | Inside | 1 | 10 | ||
| Amoxiclav | 0.375 g | Inside | 3 | 10 | ||
| Acute | Stage of early disseminated infection | The main drug is ceftriaxone | 2 g | Intramuscularly | 1 | 14 |
| Alternative drugs | ||||||
| Cefotaxime | 2 g | Intramuscularly | 3 | 14 | ||
| Penicillin | 0.5–2 million units | Intramuscularly | 8 | 14 | ||
| Doxycycline | 0.2 g | Inside | 1 | 14 | ||
| Amoxicillin | 0.5 g | Inside | 3 | 14 | ||
| Chronic course | – | The main drug is ceftriaxone | 2 g | Intramuscularly | 1 | 21 |
| Drugs of choice | ||||||
| Cefotaxime | 2 g | Intramuscularly | 3 | 21 | ||
| Penicillin | 2–3 million units | Intramuscular, intravenous | 6–8 | 21 |
In cases of mixed infection (Lyme borreliosis and tick-borne encephalitis), along with antibiotics, immunoglobulin against tick-borne encephalitis is used in calculated doses.
Detoxification therapy is carried out according to general principles. According to individual indications, vascular agents and antioxidants are used.
During the rehabilitation period, hyperbaric oxygen therapy, exercise therapy, and massage are performed. Sanatorium-resort treatment is indicated for patients in remission with a chronic course with damage to the osteoarticular and nervous systems.